Results to-date
Preclinical
BioKier has demonstrated, in Zucker Diabetic Fatty (ZDF) rats with fully developed diabetes, that infusion of model compound, butyrate, into the colon restores secretion of GLP-1 in response to oral glucose (Figure 1). This effect is identical to the one observed after RYGB surgery. Subsequently, BioKier demonstrated preclinical proof of concept in the ZDF rat, the industry standard preclinical model of type 2 diabetes, in which an oral formulation of butyrate completely prevented development of diabetes (Figure. 2). In addition to normalizing glucose, butyrate treatment improved insulin sensitivity, demonstrated by lower insulin levels required to maintain low basal glucose.
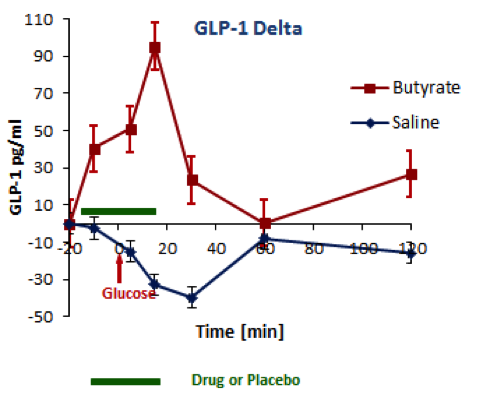
Fig. 1. Effects of butyrate infused into the colon on oral glucose-induced secretion of GLP-1 in ZDF rats. Butyrate was infused in solution in saline; saline alone acted as control. (n=5, both groups)
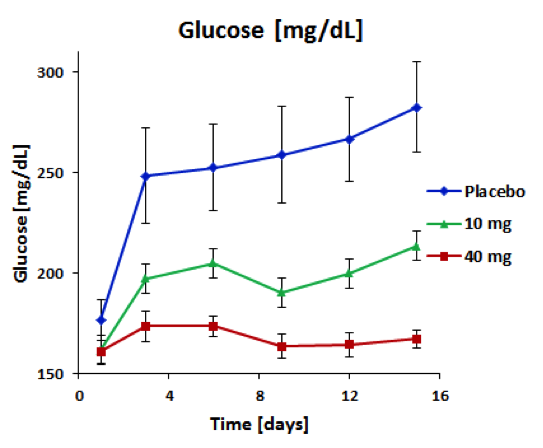
Fig. 2. Effect of treatment with sustained-release butyrate tablets targeted to the colon on basal glucose levels in 7 week-old ZDF rats (Each group n=5)
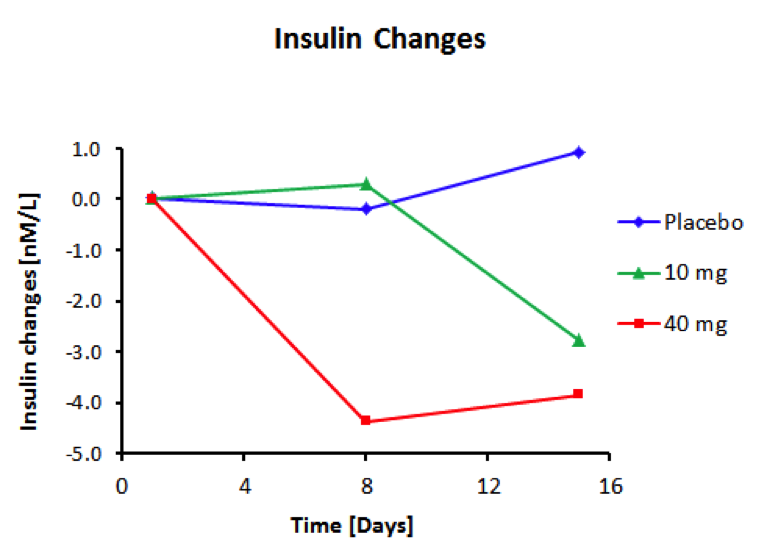
Fig. 3. Effect of treatment with sustained-release butyrate tablets targeted to the colon on basal insulin levels in 7-week-old ZDF rats (Each group N=5)
Clinical
Exploratory clinical studies of BioKier’s lead compounds in type 2 diabetes patients were conducted at Carolina Research, a gastrointestinal research clinic in Greenville, NC. In this study, single doses of butyrate and glutamine, in saline solution were administered to the sigmoid colon of type 2 diabetes patients. The study objectives were to compare the effects of the lead nutrient compounds on gut hormone, insulin, and glucose responses to an oral glucose challenge. Infusion into the sigmoid colon of 1g L-glutamine in 100 mL saline over 15 minutes caused statistically significant increases, over placebo, in secretion of both GLP-1 and insulin in response to an oral glucose challenge (Figure 4). The levels of GLP-1 and insulin secreted in the presence of L-glutamine approached those seen in a typical non-diabetes subject. The hormone responses in the presence of saline control were typical of the impaired responses seen in type 2 diabetes patients. The hormone responses after infusion of butyrate were similar to control responses. L-glutamine was therefore selected as the lead nutrient compound for further clinical development.

Fig 4. The effect of infusion of L-glutamine into the sigmoid colon on oral glucose-induced GLP-1 and insulin secretion in type 2 diabetes patients. L-glutamine (blue) or saline (red) was infused for 15 minutes (shown by the green bar) and the glucose challenge was administered at 0 min (arrow); hormones were measured (by BioAgilytix using the Meso Scale Discovery assay technology) in peripheral blood samples collected over 120 min. Data are shown as change from pretreatment levels (n=10). The dotted lines show responses from a typical non-diabetic subject for comparison. Differences between L-glutamine and placebo are statistically different for GLP-1 at 30 min and for insulin at 90 and 120 min. Differences in insulin AUC for L-glutamine and placebo are statistically significant.
To demonstrate the importance of targeting delivery of L-glutamine to the colon, the results of BioKier’s study in which 1g L-glutamine was infused directly into the colon were compared to a study in which 30g L-glutamine were administered orally in solution (Samocha-Bonet D. et al., J. Nutr. 141: 1233–1238, 2011). 1 g of L-glutamine dosed intracolonically resulted in larger augmentation of GLP-1 secretion than 30 g of orally administered L-glutamine (Figure 5). Although GLP-secretion in the BioKier study was in response to an oral glucose challenge and GLP-1 secretion in the reported study was in response to a high-carbohydrate mixed-meal challenge, this result very clearly demonstrates that much lower doses of L-glutamine are effective when delivered to the L-cell-rich colon.
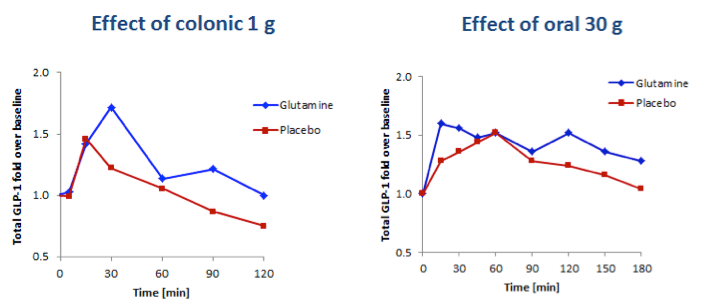
Fig 5. Comparison of the effects of 1g L-glutamine delivered to the colon and 30g L-glutamine taken orally on plasma concentrations of total GLP-1 in individuals with type 2 diabetes. Left panel, GLP-1 response to a 50-gram oral glucose challenge following infusion of 1g L-glutamine to the colon (BioKier study, n=10). Right panel, GLP-1 response to a high carbohydrate, low-fat meal challenge following oral delivery of 30g L-glutamine (J. Nutr. study, n=13).
Chronic study with BRK-013
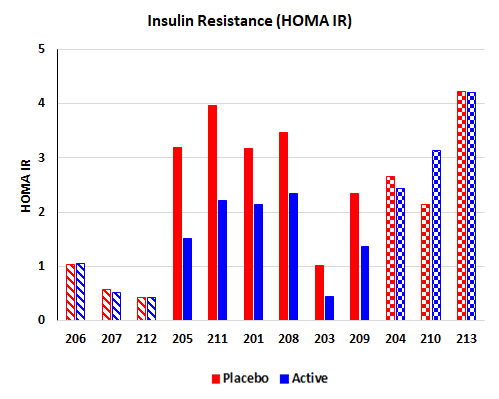
BioKier completed a four-week clinical study with BKR-013 (L-glutamine in colon-targeted, sustained-release capsules) in Type 2 diabetes patients, who were already being treated with approved anti-diabetes drugs. This study was supported by an NIH SBIR grant. The most significant and encouraging result of the study was an improvement in insulin sensitivity in patients who were insulin-resistant, after the BKR-013 treatment period compared to the placebo treatment. Of the nine (9) insulin-resistant patients, 6 demonstrated significant improvement in insulin sensitivity.